NMR spectroscopy is the most common and comprehensive technique for studying the structure of organic molecules. In a broad sense, it still works by the same principle as other spectroscopies, and that is the interaction of the molecule with certain type of energy to produce different energy states and deduce information based on these differences.
There is, however, a lot more information you can get from an NMR spectrum than what we have seen in the IR spectroscopy and Mass Spectrometry.
In this post, we won’t go into the details of how the NMR spectroscopy works and the physics behind it.
We will rather try to figure out what and where do you look for and how you understand it when analyzing an NMR spectrum?
Below is the summary of the four main pieces of information we obtain when looking at an 1H NMR spectrum.

Let’s now briefly explain what each of these represents.
The Chemical Shift
This is what we see on the x axis and it tells the energy value at which the peak appears. Just like the IR spectroscopy, different functional groups have different energy values for resonance absorption and that’s what helps us identify them. Most of the time this is going to be the first thing you look at when analyzing an NMR spectrum.
You want to know what functional groups/fragments you have – check the region where the peaks appear.
Here are the main regions in the 1H NMR spectrum that you need to know:

The energy axis is called a δ (delta) axis and the units are given in part per million (ppm). Most often the signal area for organic compounds ranges from 0-12 ppm.
Keep in mind that all the information we get about the functional groups is solely based on the protons that are in those functional groups – it is a proton 1H NMR (we also have 13C and some other nuclei NMRs). The signals in 1H NMR are originated from energy absorption and release of protons which are exposed to the energy to a different extent depending on their neighboring atoms.
We will discuss the principles of chemical shift in more detail but for now, let’s also mention the other key features of NMR.
The Number of Protons – NMR Integrals
This is another great advantage of NMR spectroscopy – not only it tells you what types of protons you have based on their environment (neighboring atoms) but it also tells you how many protons you have. And that is not just how many protons in total, you can see specifically how many protons you have for the given signal, which is coming, for example, from an aromatic ring. Depending on how many substituents are on the ring we can have a monosubstituted, disubstituted or a pentasubstituted ring which changes the number of protons on the ring and we can see that on the NMR.
This is the second point in the summary picture above labeled in blue. You find the number of protons based on the integrals:
The number of protons (it is the relative number) is given right under the integral sign. The height of the integral is proportional to the number of the protons.
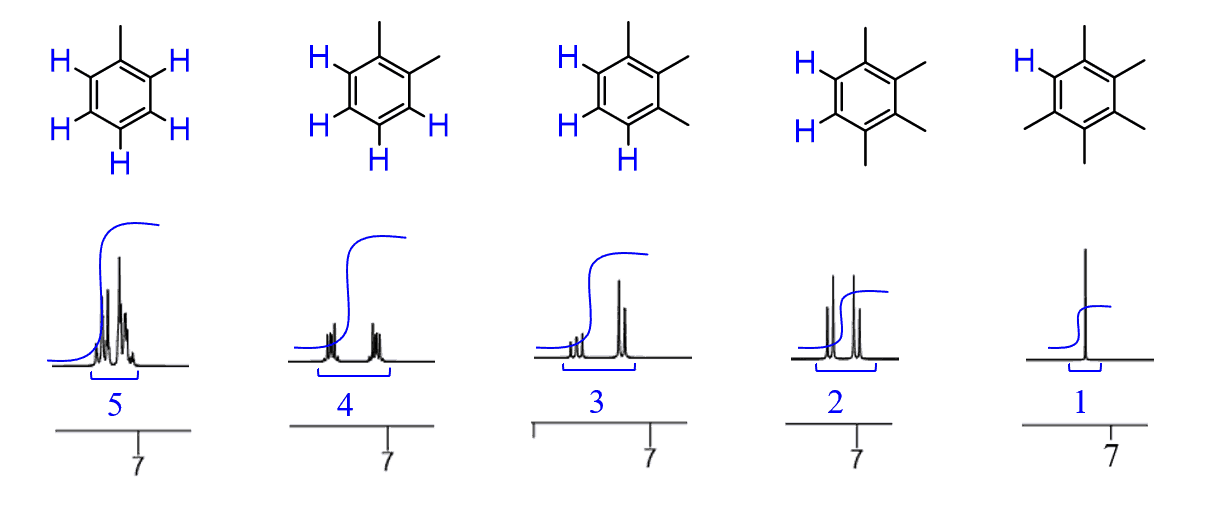
The Number of Different Protons
There are five peaks on this NMR spectrum which indicates that there are five different types of protons.

Notice that we are not talking about five protons, we are saying that there are five types of protons. For example, there are three protons in the methyl group, but they are all identical and therefore they give one signal. We don’t count the number of peaks (lines) of the signal for this purpose – they are all together representing one signal from one type of protons. The number of peaks in the signal is the multiplicity, also known as the signal splitting, which is another powerful feature of NMR spectroscopy.
Whether the protons are identical or not can relatively easily be determined by a symmetry test. Shortly put, if the two protons are reflected through a plane of symmetry or exchangeable by a symmetry axis, they give one signal:

There is, of course, more to determining the relationship of protons and whether they are identical or not since they can also enantiotopic or diastereotopic, but this gives you a good idea of what is happening.
Multiplicity – The Spin-Spin Splitting
Depending on how many neighbors it has, the signal of a given proton can be split into lines. The simplest signal consists of one line and is called a singlet, followed by the doublet, triplet, etc. A signal with more than seven lines is referred to as a multiplet.

The number of peaks is obtained by the N+1 rule, where N is the number of neighboring protons. For example, if the proton has one neighbor, it gives a doublet (1+1), if there are two adjacent protons, we get a triplet (2+1).
Again, it’s a little more complicated than this since not all neighboring protons are coupled. Signal splitting occurs only between nonequivalent protons. Nevertheless, let’s look at how nicely we can distinguish protons based on their neighboring protons that are non-equivalent to them:

Some challenges here are;
1) the neighboring protons must be equivalent to one another and not equivalent to the proton of interest
2) If the neighbors are not equivalent, then each of them splits the signal into two lines
3) Different protons plat the signal to a different extent which is measured by the J coupling constant
Each of these features we went over deserves a separate article or a few of them and we will definitely do that to make NMR a little easier!
Check Also
- NMR Chemical Shift
- NMR Chemical Shift Range and Value Table
- NMR Number of Signals and Equivalent Protons
- Homotopic Enantiotopic Diastereotopic and Heterotopic
- Homotopic Enantiotopic Diastereotopic Practice Problems
- Integration in NMR Spectroscopy
- Splitting and Multiplicity (N+1 rule) in NMR Spectroscopy
- NMR Signal Splitting N+1 Rule Multiplicity Practice Problems
- 13C NMR NMR
- DEPT NMR: Signals and Problem Solving
- NMR Spectroscopy-Carbon-Dept-IR Practice Problems
