The Use of Polymer Membranes for the Recovery of Copper, Zinc and Nickel from Model Solutions and Jewellery Waste
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
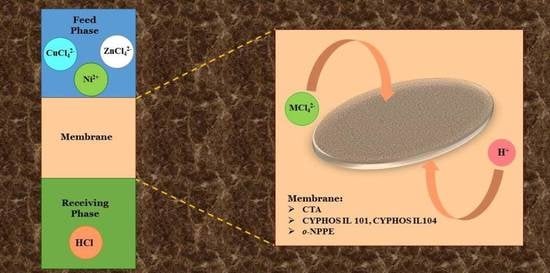
2.2. Transport of Metal Ions across PIMs
2.3. Calculation Formulae
3. Results and Discussion
3.1. Membrane Characteristics
3.2. The Effect of Carrier Concentration on Cu(II) Transport through PIMs
3.3. Effect of Cl− Ion Concentration in the Feed Phase
3.4. Separation of Copper(II), Zinc(II) and Nickel(II) Ions Using PIMs with Cyphos IL
For Cyphos IL 104: [MCl4]2− + 2 R3RP-A ↔ (R3RP)2MCl4 + 2 A−
For Cyphos IL 104: (R3RP)2MCl4 ↔ [MCl4]2− + 2 R3RP-A
3.5. Metal Ion Diffusion Coefficients
3.6. Recovery of Metal
3.7. Recovery of Metal from Jewellery Waste Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D.; Yang, Y.; Bartsch, R.A. Heavy Metal Separation with Polymer Inclusion Membranes. J. Membr. Sci. 2008, 323, 444–451. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and Transport of Metal Ions and Small Organic Compounds Using Polymer Inclusion Membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Zulkefeli, N.S.W.; Weng, S.K.; Abdul Halim, N.S. Removal of Heavy Metals by Polymer Inclusion Membranes. Curr. Pollut. Rep. 2018, 4, 84–92. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Supported Ionic Liquid and Polymer Inclusion Membranes for Metal Separation. Sep. Purif. Rev. 2022, 51, 100–116. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Nowak, Ł.; Wiśniewski, M. Removal of Zinc(II) and Iron Ions from Chloride Solutions with Phosphonium Ionic Liquids. Sep. Purif. Technol. 2012, 97, 158–163. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M.; Pyszka, I. Application of Polymer Inclusion Membranes Doped with Alkylimidazole to Separation of Silver and Zinc Ions from Model Solutions and after Battery Leaching. Materials 2020, 13, 3103. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent Trends in Extraction and Transport of Metal Ions Using Polymer Inclusion Membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Eloffy, M.G.; El-Sherif, D.M.; Abouzid, M.; Elkodous, M.A.; El-nakhas, H.S.; Sadek, R.F.; Ghorab, M.A.; Al-Anazi, A.; El-Sayyad, G.S. Proposed Approaches for Coronaviruses Elimination from Wastewater: Membrane Techniques and Nanotechnology Solutions. Nanotechnol. Rev. 2022, 11, 1–25. [Google Scholar] [CrossRef]
- Donat, R.; Eyice, M.İ.; Erden, K.E. Transportation and Extraction of Cu2+ Metal Ions from Acidic Solution by MDLM Technique. Resour. Conserv. Recycl. 2022, 179, 106124. [Google Scholar] [CrossRef]
- Staszak, M. Membrane Technologies for Sports Supplementation. Phys. Sci. Rev. 2022. [Google Scholar] [CrossRef]
- Alsobh, A.; Zin, M.M.; Vatai, G.; Bánvölgyi, S. The Application of Membrane Technology in the Concentration and Purification of Plant Extracts: A Review. Period. Polytech. Chem. Eng. 2022, 66, 394–408. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Varjani, S.; Ding, A.; Bui, X.-T.; Nguyen, D.P. Bio-Membrane Based Integrated Systems for Nitrogen Recovery in Wastewater Treatment: Current Applications and Future Perspectives. Chemosphere 2021, 265, 129076. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.M.; Garg, S.; Bajpai, S. Economic Feasibility of Arsenic Removal Using Nanofiltration Membrane: A Mini Review. Chem. Pap. 2021, 75, 4431–4444. [Google Scholar] [CrossRef]
- Kubota, F.; Kono, R.; Yoshida, W.; Sharaf, M.; Kolev, S.D.; Goto, M. Recovery of Gold Ions from Discarded Mobile Phone Leachate by Solvent Extraction and Polymer Inclusion Membrane (PIM) Based Separation Using an Amic Acid Extractant. Sep. Purif. Technol. 2019, 214, 156–161. [Google Scholar] [CrossRef]
- Fontàs, C.; Tayeb, R.; Tingry, S.; Hidalgo, M.; Seta, P. Transport of Platinum(IV) through Supported Liquid Membrane (SLM) and Polymeric Plasticized Membrane (PPM). J. Membr. Sci. 2005, 263, 96–102. [Google Scholar] [CrossRef]
- Croft, C.F.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Separation of Lanthanum(III), Gadolinium(III) and Ytterbium(III) from Sulfuric Acid Solutions by Using a Polymer Inclusion Membrane. J. Membr. Sci. 2018, 545, 259–265. [Google Scholar] [CrossRef]
- Cai, C.; Yang, F.; Zhao, Z.; Liao, Q.; Bai, R.; Guo, W.; Chen, P.; Zhang, Y.; Zhang, H. Promising Transport and High-Selective Separation of Li(I) from Na(I) and K(I) by a Functional Polymer Inclusion Membrane (PIM) System. J. Membr. Sci. 2019, 579, 1–10. [Google Scholar] [CrossRef]
- Smail, F.; Arous, O.; Amara, M.; Kerdjoudj, H. A Competitive Transport across Polymeric Membranes. Study of Complexation and Separation of Ions. Comptes Rendus Chim. 2013, 16, 605–612. [Google Scholar] [CrossRef]
- Paredes, C.; Rodríguez de San Miguel, E. Selective Lithium Extraction and Concentration from Diluted Alkaline Aqueous Media by a Polymer Inclusion Membrane and Application to Seawater. Desalination 2020, 487, 114500. [Google Scholar] [CrossRef]
- Ugur, A.; Sener, I.; Hol, A.; Alpoguz, H.K.; Elci, L. Facilitated Transport of Zn(II) and Cd(II) Ions Through Polymer Inclusion Membranes Immobilized With a Calix [4] Resorcinarene Derivative. J. Macromol. Sci. Part A 2014, 51, 611–618. [Google Scholar] [CrossRef]
- Ayyavoo, S.; Kandasamy, P.; Ramasamy, S. Removal of Mercury in Aqueous Solutions Using Tri N-Butyl Phosphate-Based Polymer Inclusion Membrane. Environ. Eng. Sci. 2022, 39, 650–661. [Google Scholar] [CrossRef]
- Sellami, F.; Kebiche-Senhadji, O.; Marais, S.; Colasse, L.; Fatyeyeva, K. Enhanced Removal of Cr(VI) by Polymer Inclusion Membrane Based on Poly(Vinylidene Fluoride) and Aliquat 336. Sep. Purif. Technol. 2020, 248, 117038. [Google Scholar] [CrossRef]
- Kaya, A.; Onac, C.; Alpoguz, H.K.; Yilmaz, A.; Atar, N. Removal of Cr(VI) through Calixarene Based Polymer Inclusion Membrane from Chrome Plating Bath Water. Chem. Eng. J. 2016, 283, 141–149. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of polymer inclusion membranes doped with 1-hexyl-4-methylimidazole for pertraction of zinc(II) and other transition metal ions. Physicochem. Probl. Miner. Process. 2015, 51, 447–460. [Google Scholar] [CrossRef]
- Ribas, T.C.F.; Croft, C.F.; Almeida, M.I.G.S.; Mesquita, R.B.R.; Kolev, S.D.; Rangel, A.O.S.S. Use of a Polymer Inclusion Membrane and a Chelating Resin for the Flow-Based Sequential Determination of Copper(II) and Zinc(II) in Natural Waters and Soil Leachates. Molecules 2020, 25, 5062. [Google Scholar] [CrossRef]
- Suah, F.B.M.; Roslan, N.A.; Dahlan, N.F.; Mohamed, N. A Use of Polymer Inclusion Membrane as Anion Exchange Membrane for Recovery of Cu(II) Ions Based on an Electrogenerative System. J. Electrochem. Soc. 2018, 165, H310. [Google Scholar] [CrossRef]
- Baczyńska, M.; Regel-Rosocka, M.; Nowicki, M.; Wiśniewski, M. Effect of the Structure of Polymer Inclusion Membranes on Zn(II) Transport from Chloride Aqueous Solutions. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Nowak, Ł.; Regel-Rosocka, M.; Marszałkowska, B.; Wiśniewski, M. Removal of Zn(II) from Chloride Acidic Solutions with Hydrophobic Quaternary Salts. Pol. J. Chem. Technol. 2010, 12, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Kogelnig, D.; Regelsberger, A.; Stojanovic, A.; Jirsa, F.; Krachler, R.; Keppler, B.K. A Polymer Inclusion Membrane Based on the Ionic Liquid Trihexyl(Tetradecyl)Phosphonium Chloride and PVC for Solid–Liquid Extraction of Zn(II) from Hydrochloric Acid Solution. Mon. Für Chem.-Chem. Mon. 2011, 142, 769–772. [Google Scholar] [CrossRef]
- Baczynska, M.; Rzelewska, M.; Regel-Rosocka, M.; Wisniewski, M. Transport of Iron Ions from Chloride Solutions Using Cellulose Triacetate Matrix Inclusion Membranes with an Ionic Liquid Carrier. Chem. Pap. 2016, 70, 172–179. [Google Scholar] [CrossRef]
- Pospiech, B. Studies on Extraction and Permeation of Cadmium(II) Using Cyphos IL 104 as Selective Extractant and Ion Carrier. Hydrometallurgy 2015, 154, 88–94. [Google Scholar] [CrossRef]
- Pospiech, B. Application of Phosphonium Ionic Liquids as Ion Carriers in Polymer Inclusion Membranes (PIMs) for Separation of Cadmium(II) and Copper(II) from Aqueous Solutions. J. Solut. Chem. 2015, 44, 2431–2447. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Liu, Y.; Zhang, C.; Chen, J. Preparation of PVDF-Based Polymer Inclusion Membrane Using Ionic Liquid Plasticizer and Cyphos IL 104 Carrier for Cr(VI) Transport. J. Membr. Sci. 2011, 372, 314–321. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alonso, M.; Lopez, F.A.; Lopez-Delgado, A. Pseudo-Emulsion Membrane Strip Dispersion (PEMSD) Pertraction of Chromium(VI) Using CYPHOS IL101 Ionic Liquid as Carrier. Environ. Sci. Technol. 2010, 44, 7504–7508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonggotgetsakul, Y.Y.; Cattrall, R.W.; Kolev, S.D. Extraction of Gold(III) from Hydrochloric Acid Solutions with a PVC-Based Polymer Inclusion Membrane (PIM) Containing Cyphos® IL 104. Membranes 2015, 5, 903–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. Recovery of Gold from Aqua Regia Digested Electronic Scrap Using a Poly(Vinylidene Fluoride-Co-Hexafluoropropene) (PVDF-HFP) Based Polymer Inclusion Membrane (PIM) Containing Cyphos® IL 104. J. Membr. Sci. 2016, 514, 274–281. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Rzelewska, M.; Baczynska, M.; Janus, M. Removal of palladium(II) from aqueous chloride solutions with cyphos phosphonium ionic liquids as metal ion carriers for liquidliquid extraction and transport across polymer inclusion membranes. Physicochem. Probl. Miner. Process. 2015, 51, 621–631. [Google Scholar] [CrossRef]
- The World Gold Council Report (WGC). Available online: https://www.gold.org/ (accessed on 1 January 2023).
- Hoyer, K.-P.; Schaper, M. Alloy Design for Biomedical Applications in Additive Manufacturing. In TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; Springer International Publishing: Cham, Switzerland, 2019; pp. 475–484. [Google Scholar]
- Jacobson, D.M.; Sangha, S.P.S.; Gales, A.; Schmid, E.E. The Development of New Silver-Free Brazing Alloys for Steel Tubular Assembly. Weld. J. 2002, 81, 149S–155S. [Google Scholar]
- ASTM B 122M; Standard Specification for Copper-Nickel-Tin Alloy, Copper-Nickel-Zinc Alloy (Nickel Silver), and Copper-Nickel Alloy Plate, Sheet, Strip, and Rolled Bar. ASTM Standards: West Conshohocken, PA, USA, 2020.
- International Copper Study Group. Available online: https://icsg.org/ (accessed on 1 January 2023).
- Hongloi, N.; Prapainainar, P.; Prapainainar, C. Review of Green Diesel Production from Fatty Acid Deoxygenation over Ni-Based Catalysts. Mol. Catal. 2022, 523, 111696. [Google Scholar] [CrossRef]
- Wan Khalit, W.N.A.; Asikin-Mijan, N.; Marliza, T.S.; Safa-Gamal, M.; Shamsuddin, M.R.; Azreena, I.N.; Saiman, M.I.; Taufiq-Yap, Y.H. One-Pot Decarboxylation and Decarbonylation Reaction of Waste Cooking Oil over Activated Carbon Supported Nickel-Zinc Catalyst into Diesel-like Fuels. J. Anal. Appl. Pyrolysis 2022, 164, 105505. [Google Scholar] [CrossRef]
- Kaika, N.; Panopoulou, C.; Anagnostopoulou, E.; Fakas, C.; Lilas, P.; Stavroulaki, D.; Papadogianakis, G. Novel Full Hydrogenation Reaction of Methyl Esters of Palm Kernel and Sunflower Oils Into Methyl Stearate Catalyzed by Rhodium, Ruthenium and Nickel Complexes of Bidentate Hexasulfonated o-Phenylendiphosphite Ligands. Catal. Lett. 2019, 149, 580–590. [Google Scholar] [CrossRef]
- Biryukov, A.I.; Kozaderov, O.A.; Zakharievich, D.A.; Galin, R.G.; Burmistrov, L.O.; Batmanova, T.V.; Zhivulin, V.E. Corrosion of Diffusion Iron–Zinc Coatings (δ-Phase) in an Alkaline Medium. Int. J. Corros. Scale Inhib. 2021, 10, 1677–1688. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Croy, J.R.; Lee, E.; Gutierrez, A.; He, M.; Park, J.S.; Yonemoto, B.T.; Long, B.R.; Blauwkamp, J.D.; Johnson, C.S.; et al. The Quest for Manganese-Rich Electrodes for Lithium Batteries: Strategic Design and Electrochemical Behavior. Sustain. Energy Fuels 2018, 2, 1375–1397. [Google Scholar] [CrossRef]
- Larcin, J.; Maskell, W.C.; Tye, F.L. Leclanché Cell Investigations. Part II: Zinc Potential as a Tool for Studying Intermittent Discharge. Electrochim. Acta 1998, 44, 191–199. [Google Scholar] [CrossRef]
- Cho, M.; Kwon, E.; Park, K. Design and Control of Pneumatic System for Recycling Classification of Non-Ferrous Metals. Int. J. Precis. Eng. Manuf. -Green Technol. 2022, 9, 567–575. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Z.; Wang, C.; Pang, Q.; Hua, L. Research on the Process of Small Sample Non-Ferrous Metal Recognition and Separation Based on Deep Learning. Waste Manag. 2021, 126, 266–273. [Google Scholar] [CrossRef]
- Pospiech, B.; Kujawski, W. Ionic Liquids as Selective Extractants and Ion Carriers of Heavy Metal Ions from Aqueous Solutions Utilized in Extraction and Membrane Separation. Rev. Chem. Eng. 2015, 31, 179–191. [Google Scholar] [CrossRef]
- Kumar, A.; Haddad, R.; Sastre, A.M. Integrated Membrane Process for Gold Recovery from Hydrometallurgical Solutions. AIChE J. 2001, 47, 328–340. [Google Scholar] [CrossRef]
- Imdad, S.; Dohare, R.K. A Critical Review On Heavy Metals Removal Using Ionic Liquid Membranes From The Industrial Wastewater. Chem. Eng. Process.—Process Intensif. 2022, 173, 108812. [Google Scholar] [CrossRef]
- Kadokawa, J. Ionic Liquids—New Aspects for the Future; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Lu, J.; He, K.; Wang, Y.; Chen, G.; Weng, H.; Lin, M. An Effective Process for the Separation of U(VI), Th(IV) from Rare Earth Elements by Using Ionic Liquid Cyphos IL 104. Chin. Chem. Lett. 2022, 33, 3422–3428. [Google Scholar] [CrossRef]
- Wellens, S.; Thijs, B.; Binnemans, K. An Environmentally Friendlier Approach to Hydrometallurgy: Highly Selective Separation of Cobalt from Nickel by Solvent Extraction with Undiluted Phosphonium Ionic Liquids. Green Chem. 2012, 14, 1657–1665. [Google Scholar] [CrossRef] [Green Version]
- Wiecka, Z.; Rzelewska-Piekut, M.; Regel-Rosocka, M. Recovery of Platinum Group Metals from Spent Automotive Converters by Leaching with Organic and Inorganic Acids and Extraction with Quaternary Phosphonium Salts. Sep. Purif. Technol. 2022, 280, 119933. [Google Scholar] [CrossRef]
- Rios-Vera, R.M.; Chagnes, A.; Hernández-Perales, L.; Martínez-Rodríguez, D.E.; Navarro-Segura, D.L.; Gaillon, L.; Sirieix-Plénet, J.; Rizzi, C.; Rollet, A.L.; Avila-Rodriguez, M.; et al. Trihexyl(Tetradecyl)Phosphonium Bis-2,4,4-(Trimethylpentyl)Phosphinate Micellar Behavior in the Extraction of Ag(I) from Acidic Nitrate Media. J. Mol. Liq. 2022, 358, 119132. [Google Scholar] [CrossRef]
- St John, A.M.; Cattrall, R.W.; Kolev, S.D. Determination of the Initial Flux of Polymer Inclusion Membranes. Sep. Purif. Technol. 2013, 116, 41–45. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of Metal Species by Supported Liquid Membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Kislik, V.S. (Ed.) Liquid Membranes; Elsevier: Amsterdam, The Netherlands, 2010; p. iv. ISBN 978-0-444-53218-3. [Google Scholar]
- Arous, O.; Amara, M.; Kerdjoudj, H. Synthesis and Characterization of Cellulose Triacetate and Poly(Ethylene Imine) Membranes Containing a Polyether Macrobicyclic: Their Application to the Separation of Copper(II) and Silver(I) Ions. J. Appl. Polym. Sci. 2004, 93, 1401–1410. [Google Scholar] [CrossRef]
- Mahanty, B.N.; Mohapatra, P.K.; Raut, D.R.; Das, D.K.; Behere, P.G.; Afzal, M.; Verboom, W. Polymer Inclusion Membrane Containing a Tripodal Diglycolamide (T-DGA): Characterization and Sorption Isotherm Studies. J. Environ. Chem. Eng. 2016, 4, 1826–1838. [Google Scholar] [CrossRef]
- Kumar, R.; Pandey, A.K.; Sharma, M.K.; Panicker, L.V.; Sodaye, S.; Suresh, G.; Ramagiri, S.V.; Bellare, J.R.; Goswami, A. Diffusional Transport of Ions in Plasticized Anion-Exchange Membranes. J. Phys. Chem. B 2011, 115, 5856–5867. [Google Scholar] [CrossRef]
- Arous, O.; Saoud, F.; Amara, M.; Kerdjoudj, H. Efficient Facilitated Transport of Lead and Cadmium Across a Plasticized Triacetate Membrane Mediated by D2EHPA and TOPO. Mater. Sci. Appl. 2011, 2, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Cooper, W.C. Nickel Speciation in Aqueous Chloride Solutions. Electrochim. Acta 1996, 41, 1549–1560. [Google Scholar] [CrossRef]
- Tor, A.; Arslan, G.; Muslu, H.; Celiktas, A.; Cengeloglu, Y.; Ersoz, M. Facilitated Transport of Cr(III) through Polymer Inclusion Membrane with Di(2-Ethylhexyl)Phosphoric Acid (DEHPA). J. Membr. Sci. 2009, 329, 169–174. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of Hydrophobic Alkylimidazoles in the Separation of Non-Ferrous Metal Ions across Plasticised Membranes—A Review. Membranes 2020, 10, 331. [Google Scholar] [CrossRef] [PubMed]









| Name | Cu | Ni | Zn | Pb | Fe | Mn | Sn | Other |
|---|---|---|---|---|---|---|---|---|
| Concentration [%] | 63–66 | 11–13 | 20–25 | 0.03 | 0.3 | 0.5 | 0.03 | 0.2 |
| Physical Properties | CYPHOS 101 | CYPHOS 104 |
|---|---|---|
| Formula | C32H68ClP | C48H102O2P |
| Representation | R3RP−Cl | R3RP−A |
| Molecular mass g/mol | 519.31 | 773.27 |
| Density at 20 °C, g/cm3 | 0.882 | 0.892 |
| Viscosity at 25 °C, cP | 1824 | 707 |
| Colour and form | Colourless liquid | Dark brown liquid |
| No. | Formula | |
|---|---|---|
| (1) | c0—initial metal ion concentration (M); ct—metal ion concentration at a given time in the feed phase (M); k is the rate constant (s−1), which could be evaluated by plotting (C0 − Ct) against time; t—time of transport (s) | |
| (2) | P—permeability coefficient; V—volume of the aqueous feed phase (m3); A—effective area of the membrane (m2) | |
| (3) | J0—initial flux (mol/sm2) | |
| (4) | S—selectivity coefficient | |
| (5) | RF—recovery coefficient | |
| (6) | Ra—surface average roughness; Zj—the current surface height value; N—number of points measured Rq—root mean square roughness | |
| (7) | ||
| (8) | D0—diffusion coefficients of metal ions; d—thickness of membrane; Δ0 could be evaluated by plotting (C0 − Ct) against time |
| Polymer Inclusion Membranes with Cyphos | ||
|---|---|---|
| Carrier | Cyphos 101 | Cyphos 104 |
| Average roughness (Ra, nm) | 3.32 ± 0.02 | 2.67 ± 0.02 |
| Mean square roughness (Rq, nm) | 4.48 ± 0.04 | 2.54 ± 0.03 |
| CTA, wt. % | Cyphos 101, wt. % | ONPPE, wt. % | K × 103, s−1 | d, μm |
|---|---|---|---|---|
| 100 | - | - | 0.42 | 22.5 ± 0.5 |
| 80 | 15 | 5 | 0.76 | 25.1 ± 0.6 |
| 60 | 35 | 5 | 1.32 | 33.2 ± 0.4 |
| 40 | 55 | 5 | 7.23 | 38.1 ± 0.5 |
| 35 | 60 | 5 | 5.06 | 45.7 ± 0.04 |
| CTA, wt. % | Cyphos 104, wt. % | ONPPE, wt. % | K × 103, s−1 | d, μm |
|---|---|---|---|---|
| 80 | 15 | 5 | 0.55 | 31.4 ± 0.8 |
| 60 | 35 | 5 | 1.06 | 39.3 ± 0.5 |
| 40 | 55 | 5 | 5.87 | 44.7 ± 0.8 |
| 35 | 60 | 5 | 2.38 | 52.1 ± 0.05 |
| Carrier | Metal Ion(II) | Initial Flux J0 × 106, mol/s·m2 | SCu(II)/M(II) |
|---|---|---|---|
| Cyphos IL101 | Cu(II) | 4.87 | Cu(II) > Zn(II) > Ni(II) 2.3 10.8 |
| Zn(II) | 2.12 | ||
| Ni(II) | 0.45 | ||
| Cyphos IL104 | Cu(II) | 3.75 | Cu(II) > Zn(II) > Ni(II) 1.9 9.1 |
| Zn(II) | 1.96 | ||
| Ni(II) | 0.41 |
| Carrier | Metal Ion(II) | Δ0, s/cm | D0, cm2/s |
|---|---|---|---|
| Cyphos IL 101 | Cu(II) | 228.14 | 1.67 × 10−7 |
| Zn(II) | 43.54 | 8.75 × 10−7 | |
| Ni(II) | - | - | |
| Cyphos IL 104 | Cu(II) | 429.81 | 1.04 × 10−7 |
| Zn(II) | 86.96 | 5.14 × 10−8 | |
| Ni(II) | - | - |
| Metal Ions | Initial Flux, J0 × 106, mol/s·m2 | SCu(II)/M(II) | RF, % |
|---|---|---|---|
| Cu(II) | 5.53 | Cu(II) > Zn(II) > Ni(II) 2.46 553 | 90 |
| Zn(II) | 1.84 | 35 | |
| Ni(II) | 0.01 | >0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzymińska-Lenarcik, E.; Pyszka, I.; Urbaniak, W. The Use of Polymer Membranes for the Recovery of Copper, Zinc and Nickel from Model Solutions and Jewellery Waste. Polymers 2023, 15, 1149. https://doi.org/10.3390/polym15051149
Radzymińska-Lenarcik E, Pyszka I, Urbaniak W. The Use of Polymer Membranes for the Recovery of Copper, Zinc and Nickel from Model Solutions and Jewellery Waste. Polymers. 2023; 15(5):1149. https://doi.org/10.3390/polym15051149
Chicago/Turabian StyleRadzymińska-Lenarcik, Elżbieta, Ilona Pyszka, and Włodzimierz Urbaniak. 2023. "The Use of Polymer Membranes for the Recovery of Copper, Zinc and Nickel from Model Solutions and Jewellery Waste" Polymers 15, no. 5: 1149. https://doi.org/10.3390/polym15051149