- A famously obsessed chemist has studied the size and shape of the crannies on Mentos candies.
- He's not alone—scientists, enthusiasts, children, and literally everyone else love this experiment.
- The micro-craggy surface of the candies is one reason why they explode so pleasingly.
Dropping Mentos candies into Diet Coke is one of the most popular urban legends and home experiments. If you eat them together, it’s supposed to kill you (it doesn’t, of course ... but we still don’t recommend it for comfort), and when you drop a Mentos into a bottle of Diet Coke, it initiates a chemical reaction that creates a volcano of foam.
Tom Kuntzleman, a.k.a. Tom Technetium on YouTube, is obsessed with the experiment.
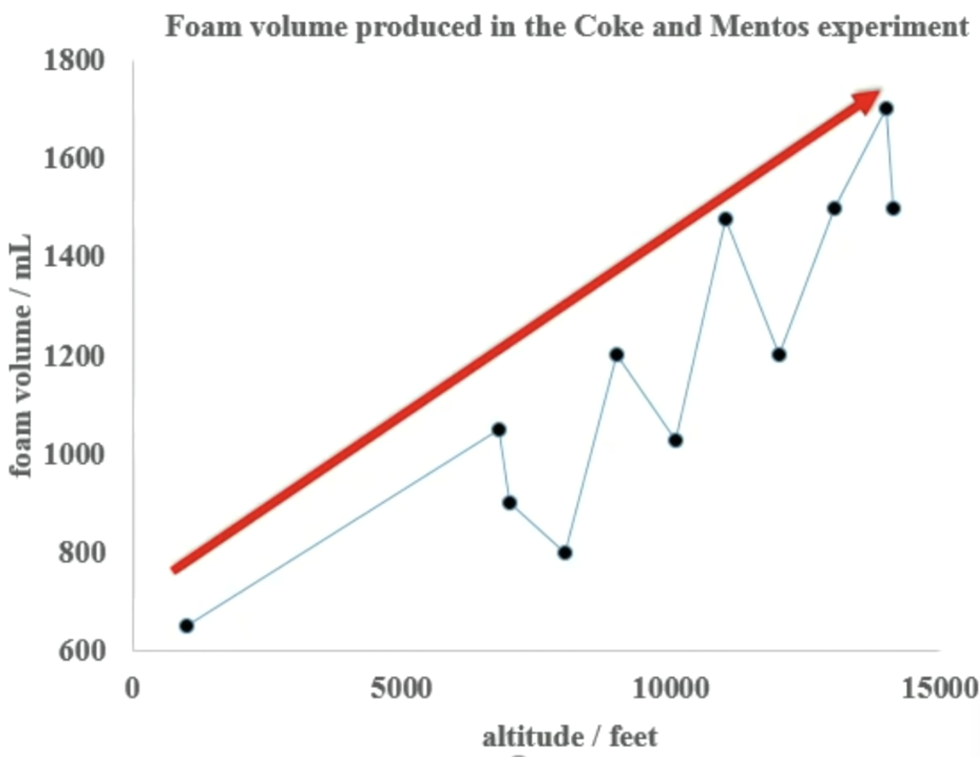
“I’ve performed this experiment thousands of times,” he says in the video, “and every time I try it, I seem to learn something new.” That’s what happened when he performed the experiment at a friend’s home in super high-elevation Colorado, where, he says, the results were much more intense than he’d noticed closer to sea level.
Today, Kuntzleman has published a new paper further investigating those results.
The paper discusses the way bubbles nucleate—the same way airplanes seed clouds to make it rain or snow—due to the microscopic texture on the surface of Mentos candies. The results, and the tiny variations between candies, explain why his experiment had overall results in one direction, but with high variation among individual data.
To do a controlled experiment, he kept two bottles from a 10-pack of Diet Cokes at his campus in Michigan and sent the rest to Colorado. There, his friend climbed higher and higher up a mountain, stopping every so often to conduct another experiment.
The air temperature varies a little based on sunshine and other factors, and of course, Mentos candies themselves have some tiny amount of variance even among consistently manufactured goods—the nucleation sites that Kuntzleman has now demonstrated in his research. But overall, the trend is that the higher altitudes mean a much higher volume of foam during the experiment.
The measuring technique is also really inventive. Kuntzleman has turned an empty 2-liter soda bottle into a graduated non-cylinder by cutting off the bottom, marking off milliliters by units of 25, and attaching a “tornado tube”: that cylinder of plastic you use to connect two soda bottles in order to make an hourglass-shaped tornado. Then he uses a narrow tube to make sure the Mentos drops directly into the soda with no obstacles.
In the video, Kuntzleman says the experiment works with Coke or any carbonated beverage. That’s true to an extent, but diet sodas form a larger volume of foam made of tinier bubbles because of the chemical qualities of sugar or corn syrup versus artificial sweeteners. If you can safely obtain some Mentos, give it a try.

Caroline Delbert is a writer, avid reader, and contributing editor at Pop Mech. She's also an enthusiast of just about everything. Her favorite topics include nuclear energy, cosmology, math of everyday things, and the philosophy of it all.